Olin research shows US does, indeed, have capacity to manufacture essential drugs
- September 29, 2022
- By Jill Young Miller
- 3 minute read
On September 12, President Joe Biden signed an executive order to launch a National Biotechnology and Biomanufacturing Initiative, noting the United States relies too heavily on foreign materials and foreign bioproduction. Off-shoring of critical industries threatens US ability to access materials like important chemicals and active pharmaceutical ingredients.
Consider the prescriptions you or your loved ones need for high blood pressure, infections or other ailments. Chances are, no manufacturing source exists in the United States for critical generic drugs or their active ingredients.
In fact, in 2021 the White House sounded the alarm about vulnerabilities in the pharmaceutical supply chain that has led to shortages of critical medicines the Food and Drug Administration deems “essential.” A White House report proclaimed, “The disappearance of domestic production of essential antibiotics impairs our ability to counter threats ranging from pandemics to bio-terrorism, as emphasized by the FDA’s analysis of supply chains for active pharmaceutical ingredients.”
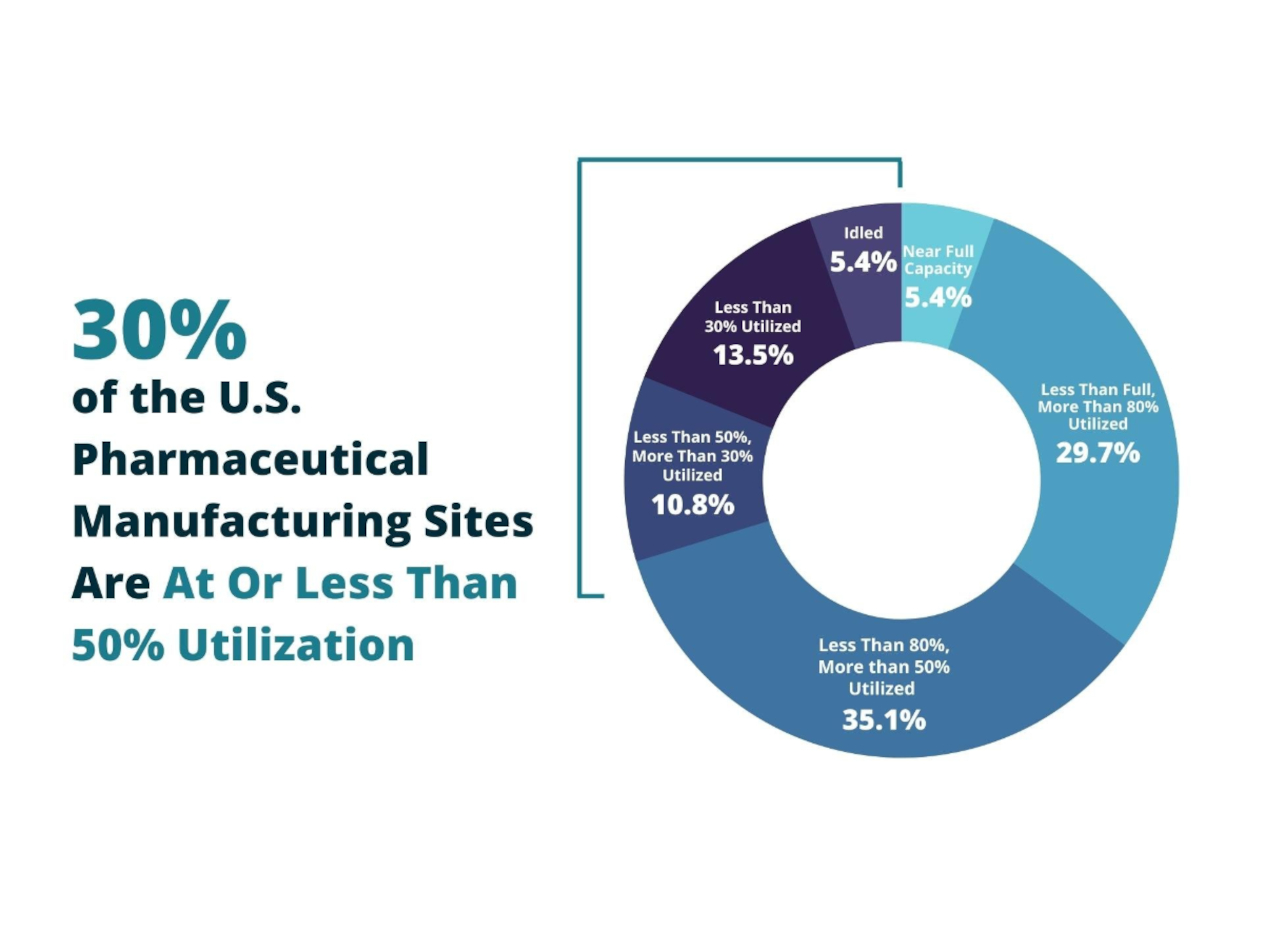
The problem? It seemed that insufficient US manufacturing capacity due to offshoring was largely to blame. But new research from the Center for Analytics and Business Insights, at Olin Business School at Washington University, finds the US does, indeed, have the capacity to make the nation’s most essential and critical drugs—yet most of the capacity is sitting idle.
Report fills key data gap
The CABI report fills a crucial gap in available industry data: “US Generic Pharmaceutical Manufacturer Available Capacity Research Survey.”

“We addressed of a significant blind spot, which was the understanding of available capacity in the United States to build supply chain resiliency,” said Anthony Sardella, author of the CABI report, senior research advisor for CABI and Olin adjunct lecturer.
“Our results were quite surprising. Fifty percent of available capacity is not utilized,” he said. “The number was stunning.”
On September 14, the Biden administration revealed it will invest more $2 billion into biotech and biomanufacturing efforts, with $1 billion from the Department of Defense for manufacturing infrastructure in the US.
30 billion more doses possible
Last year, the generic pharmaceutical industry made headlines when it announced the closure of several U.S. manufacturing plants. Why? In part because of lower offshore operating costs and labor rates, intense pricing pressure and steadily growing dependence on offshore sources for raw materials.
“How do we account for this incongruency?” Sardella asks. He and his team surveyed 37 U.S. generic pharmaceutical manufacturing sites. They found the sites are producing at just half of their production capacity annually, with an aggregate excess capacity of nearly 50%. In fact, only two of the 37 manufacturing sites are producing at full capacity.
If the sites got up and running, nearly 30 billion additional doses of essential and critical medicines could be produced in the US without incurring the expense of building new manufacturing plants and shorten the time to make generic medicines available from domestic sources, according to the report.
In a nutshell, the report recommends the following:
- Repurpose idle sites to enable manufacturing to address shortages, increase supply-chain resiliency and build supplies within 24-36 months.
- Continue current federal funding efforts for advanced manufacturing technologies to reduce production costs, create new workforce opportunities and increase the economic sustainability of US drug manufacturing.
Research aims to foster national policy
Sardella, who focused his research on issues at the intersection of business, government and society, will present the results of the paper on October 4 to the National Press Club in Washington, DC, to provide support for policy considerations and initiatives to strengthen US drug manufacturing sustainability.
The next step for CABI is its new paper, in progress, about how to model funding initiatives that de-risks the adoption of new, advanced manufacturing technologies, such as continuous-flow chemistry, to boost production.
CABI has been researching the drug shortage issue over the past couple of years.
Media inquiries
For assistance with media inquiries and to find faculty experts, please contact Washington University Marketing & Communications.
Monday–Friday, 8:30 to 5 p.m.
Sara Savat
Senior News Director, Business and Social Sciences
